

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 11:00
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2
You know the right answer?
The voltage generated by the zinc concentration cell described by the line notation Zn ( s ) ∣ ∣ Zn...
Questions


Mathematics, 05.05.2020 15:56

English, 05.05.2020 15:56

Mathematics, 05.05.2020 15:56


Social Studies, 05.05.2020 15:56

Chemistry, 05.05.2020 15:56



Spanish, 05.05.2020 15:56

Mathematics, 05.05.2020 15:56

Chemistry, 05.05.2020 15:56



Social Studies, 05.05.2020 15:56


History, 05.05.2020 15:56

Spanish, 05.05.2020 15:56


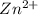 ion at cathode is 0.704 M
ion at cathode is 0.704 M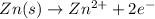
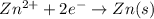
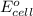 will be equal to zero.
will be equal to zero.![E_{cell}=E^o_{cell}-\frac{0.0592}{n}\log \frac{[Zn^{2+}_{anode}]}{[Zn^{2+}_{cathode}]}](/tpl/images/0536/7525/d92a3.png)
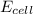 = 25.0 mV = 0.025 V (Conversion factor: 1 V = 1000 mV)
= 25.0 mV = 0.025 V (Conversion factor: 1 V = 1000 mV)![[Zn^{2+}_{cathode}]](/tpl/images/0536/7525/b27eb.png) = ? M
= ? M![[Zn^{2+}_{anode}]](/tpl/images/0536/7525/66f39.png) = 0.100 M
= 0.100 M![0.025=0-\frac{0.0592}{2}\log \frac{0.100}{[Zn^{2+}_{anode}]}](/tpl/images/0536/7525/4f785.png)
![[Zn^{2+}_{anode}]=0.704M](/tpl/images/0536/7525/377d9.png)


