

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
Determine the theoretical yield of H2S (in moles) if 4.0 molAl2S3 and 4.0 mol H2O are reacted accord...
Questions

Health, 15.04.2021 04:20

Computers and Technology, 15.04.2021 04:20

Mathematics, 15.04.2021 04:20

Mathematics, 15.04.2021 04:20


Advanced Placement (AP), 15.04.2021 04:20


Mathematics, 15.04.2021 04:20

English, 15.04.2021 04:20


Mathematics, 15.04.2021 04:20

Mathematics, 15.04.2021 04:20

Social Studies, 15.04.2021 04:20



Mathematics, 15.04.2021 04:20


Mathematics, 15.04.2021 04:20


Mathematics, 15.04.2021 04:20

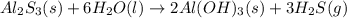
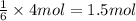 of aluminum sulfide
of aluminum sulfide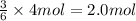 of hydrogen sulfide
of hydrogen sulfide

