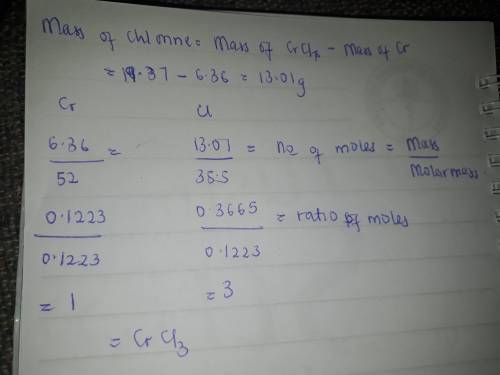Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
When 6.36 g of metallic chromium is heated with elemental chlorine gas, 19.37 g of a chromium chlori...
Questions

Mathematics, 26.03.2021 01:50



English, 26.03.2021 01:50

Mathematics, 26.03.2021 01:50


Chemistry, 26.03.2021 01:50


Physics, 26.03.2021 01:50

Mathematics, 26.03.2021 01:50


Social Studies, 26.03.2021 01:50


Mathematics, 26.03.2021 01:50


Mathematics, 26.03.2021 01:50




Mathematics, 26.03.2021 01:50