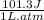Chemistry, 07.03.2020 00:09 makennskyee7114
Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 10.0 atm. Calculate the work, w , if the gas expands against a constant external pressure of 1.00 atm to a final volume of 10.0 L.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

You know the right answer?
Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 10.0 atm. Calculate...
Questions








History, 31.05.2021 15:20

World Languages, 31.05.2021 15:30



Mathematics, 31.05.2021 15:30