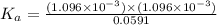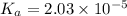Chemistry, 07.03.2020 00:05 jessicaortiz6
Using the average initial pH of your acetic acid solutions (2.96), and the average molarity of those solutions(.0602), calculate a value of Ka for acetic acid. Report your result to 3 significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

You know the right answer?
Using the average initial pH of your acetic acid solutions (2.96), and the average molarity of those...
Questions

Mathematics, 02.06.2021 04:20


Mathematics, 02.06.2021 04:20



Physics, 02.06.2021 04:30

Mathematics, 02.06.2021 04:30

Mathematics, 02.06.2021 04:30


Mathematics, 02.06.2021 04:30

Mathematics, 02.06.2021 04:30


Mathematics, 02.06.2021 04:30


Chemistry, 02.06.2021 04:30


Health, 02.06.2021 04:30



Chemistry, 02.06.2021 04:30

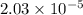
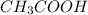 .
.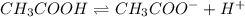
![pH=-\log [H^+]](/tpl/images/0536/6580/37e81.png)
![2.96=-\log [H^+]](/tpl/images/0536/6580/04be1.png)
![[H^+]=1.096\times 10^{-3}M](/tpl/images/0536/6580/640ad.png)
![[H^+]=[CH_3COO^-]=1.096\times 10^{-3}M](/tpl/images/0536/6580/4b8c0.png)
![[CH_3COOH]=0.0602-(1.096\times 10^{-3})=0.0591M](/tpl/images/0536/6580/19210.png)
![K_a=\frac{[CH_3COO^-][H^+]}{[CH_3COOH]}](/tpl/images/0536/6580/9c35d.png)