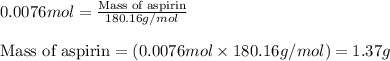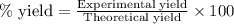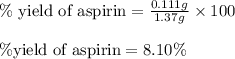Chemistry, 07.03.2020 00:10 peperivera2652738
A student measures out exactly 0.105 g of salicylic acid and runs the experiment as dictated in the lab manual. They obtain 0.111 g of aspirin. What is the percent yield for their reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2


Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
A student measures out exactly 0.105 g of salicylic acid and runs the experiment as dictated in the...
Questions


Mathematics, 20.03.2020 21:09

Biology, 20.03.2020 21:09

Mathematics, 20.03.2020 21:09

History, 20.03.2020 21:09



Mathematics, 20.03.2020 21:10




Mathematics, 20.03.2020 21:10


Mathematics, 20.03.2020 21:10

Computers and Technology, 20.03.2020 21:10

Social Studies, 20.03.2020 21:10

Mathematics, 20.03.2020 21:10



Mathematics, 20.03.2020 21:11

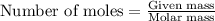 .....(1)
.....(1)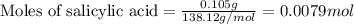
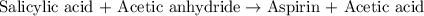
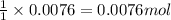 of aspirin
of aspirin