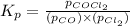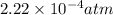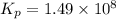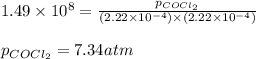Chemistry, 07.03.2020 00:09 youngbeauty17
The Kp for the reaction below is 1.49 × 108 at 100.0°C:CO(g) + Cl2(g) → COCl2(g)In an equilibrium mixture of the three gases, PCO = PCl2 = 2.22 × 10-4 atm. The partial pressure of the product, phosgene (COCl2), is atm.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2


You know the right answer?
The Kp for the reaction below is 1.49 × 108 at 100.0°C:CO(g) + Cl2(g) → COCl2(g)In an equilibrium mi...
Questions



Mathematics, 01.02.2020 00:02




History, 01.02.2020 00:02

History, 01.02.2020 00:02




Mathematics, 01.02.2020 00:02


History, 01.02.2020 00:02

Mathematics, 01.02.2020 00:02

Mathematics, 01.02.2020 00:02



Mathematics, 01.02.2020 00:02

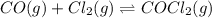
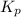 for above equation follows:
for above equation follows: