
Chemistry, 07.03.2020 00:34 Candieboo4006
Ammonium hydrogen sulfide decomposes according to the following reaction, for which Kp = 0.11 at 250ºC: NH4HS(s) H2S(g) + NH3(g) If 55.0 g of NH4HS(s) is placed in a sealed 5.0-L container, what is the partial pressure of NH3(g) at equilibrium

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1


Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
Ammonium hydrogen sulfide decomposes according to the following reaction, for which Kp = 0.11 at 250...
Questions


Mathematics, 08.10.2021 07:30

History, 08.10.2021 07:30

Mathematics, 08.10.2021 07:40


Mathematics, 08.10.2021 07:40


Chemistry, 08.10.2021 07:40

Mathematics, 08.10.2021 07:40

Mathematics, 08.10.2021 07:40

Mathematics, 08.10.2021 07:40

English, 08.10.2021 07:40

Chemistry, 08.10.2021 07:40

Mathematics, 08.10.2021 07:40

Advanced Placement (AP), 08.10.2021 07:40


Mathematics, 08.10.2021 07:40



Business, 08.10.2021 07:40

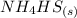 ⇄
⇄ 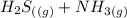
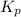 = 0.11
= 0.11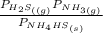
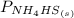 = 1 since it is solid and solid and it is known that any solid has uniformity at equilibrium.
= 1 since it is solid and solid and it is known that any solid has uniformity at equilibrium.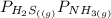
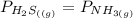
![[x][x]](/tpl/images/0536/8008/ce5d5.png)
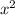
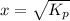
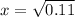
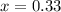 atm
atm

