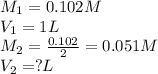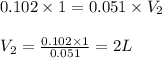Chemistry, 07.03.2020 00:59 jerikkaharris7057
You make 1.000 L of an aqueous solution that contains 35.0 g of sucrose (C12H22O11).
A) What is the molarity of sucrose in this solution? (I got 0.102 M for Part A) I just can't figure out part B.
B) How many liters of water would you have to add to this solution to reduce the molarity you calculated in Part A by a factor of two?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3
You know the right answer?
You make 1.000 L of an aqueous solution that contains 35.0 g of sucrose (C12H22O11).
A)...
A)...
Questions

Chemistry, 02.01.2021 16:50

Physics, 02.01.2021 16:50





English, 02.01.2021 16:50

Chemistry, 02.01.2021 16:50





Mathematics, 02.01.2021 16:50

Biology, 02.01.2021 16:50



Geography, 02.01.2021 16:50


Mathematics, 02.01.2021 16:50

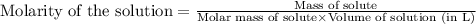
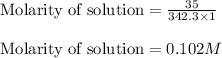
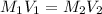
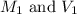 are the molarity and volume of the concentrated sucrose solution
are the molarity and volume of the concentrated sucrose solution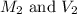 are the molarity and volume of diluted sucrose solution
are the molarity and volume of diluted sucrose solution