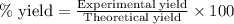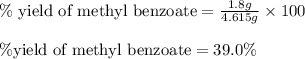Chemistry, 07.03.2020 01:27 Nevaeh3700
Consider the Fischer ester synthesis of methyl benzoate from benzoic acid and methanol in the presence of sulfuric acid as a catalyst. A reaction was performed in which 3.8 g of benzoic acid was reacted with excess methanol to make 1.8 g of methyl benzoate. Calculate the theoretical yield and percent yield for this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1



Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
Consider the Fischer ester synthesis of methyl benzoate from benzoic acid and methanol in the presen...
Questions








Mathematics, 08.01.2021 18:00

Physics, 08.01.2021 18:00





Chemistry, 08.01.2021 18:00

Mathematics, 08.01.2021 18:00

Physics, 08.01.2021 18:00

Mathematics, 08.01.2021 18:00

History, 08.01.2021 18:00

Mathematics, 08.01.2021 18:00

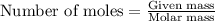 .....(1)
.....(1)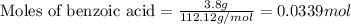
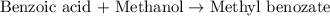
 of methyl benzoate
of methyl benzoate