Problem PageQuestion Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 75.0L tank with 23. mol of ammonia gas at 48.°C. He then raises the temperature, and when the mixture has come to equilibrium measures the amount of hydrogen gas to be 21. mol. Calculate the concentration equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Problem PageQuestion Ammonia will decompose into nitrogen and hydrogen at high temperature. An indus...
Questions





Mathematics, 14.07.2019 14:00

Business, 14.07.2019 14:00


Mathematics, 14.07.2019 14:00



Mathematics, 14.07.2019 14:00



History, 14.07.2019 14:00


English, 14.07.2019 14:00

Mathematics, 14.07.2019 14:00



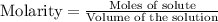
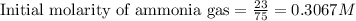
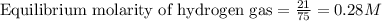
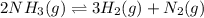
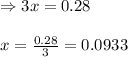
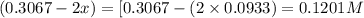
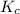 for above equation follows:
for above equation follows:![K_c=\frac{[H_2]^3[N_2]}{[NH_3]^2}](/tpl/images/0536/9367/5cab9.png)