

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

You know the right answer?
A weak acid is titrated with 0.1236 M NaOH. From the titration curve you determine that the equivale...
Questions

Mathematics, 19.11.2020 02:40

Social Studies, 19.11.2020 02:40

Mathematics, 19.11.2020 02:40


Mathematics, 19.11.2020 02:40





Mathematics, 19.11.2020 02:40


Mathematics, 19.11.2020 02:40

Spanish, 19.11.2020 02:40

English, 19.11.2020 02:40

Mathematics, 19.11.2020 02:40

Mathematics, 19.11.2020 02:40

History, 19.11.2020 02:40


Arts, 19.11.2020 02:40

Physics, 19.11.2020 02:40

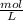 × 12.43 mL
× 12.43 mL

