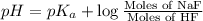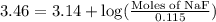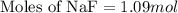Chemistry, 07.03.2020 02:10 witerose701
How many moles of solid NaF would have to be added to 1.0 L of 0.115 M HF solution to achieve a buffer of pH 3.46? Assume there is no volume change. Ka for HF = 7.2 × 10 –4

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2

Chemistry, 23.06.2019 16:30
All chemical reactions use reactants in a specific proportion or stoichiometry to form products. the reactant regulates the amount of products produced. a) excess b) limiting c) proportional d) stoichiometric
Answers: 1
You know the right answer?
How many moles of solid NaF would have to be added to 1.0 L of 0.115 M HF solution to achieve a buff...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01








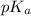 .
.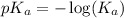
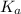 in this expression, we get:
in this expression, we get: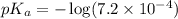
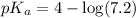
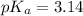
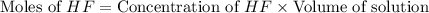
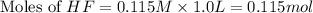
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0537/0697/e961a.png)