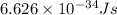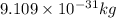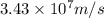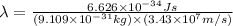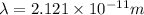Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2


Chemistry, 23.06.2019 13:30
What is matter? a. anything that has mass and takes up space b. something that has volume and takes up space. c. things that have energy and take up space d. things that take up space but don't have mass
Answers: 2
You know the right answer?
What is the wavelength of an electron with a mass of 9.109×10−31 kg and a velocity of 3.43×107 ms? U...
Questions

Mathematics, 17.10.2020 22:01


Social Studies, 17.10.2020 22:01


Mathematics, 17.10.2020 22:01

Mathematics, 17.10.2020 22:01


English, 17.10.2020 22:01


Mathematics, 17.10.2020 22:01




Mathematics, 17.10.2020 22:01

Arts, 17.10.2020 22:01

Biology, 17.10.2020 22:01




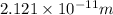
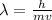
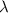 = De-Broglie's wavelength = ?
= De-Broglie's wavelength = ?