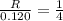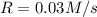Chemistry, 07.03.2020 02:49 minecraft37385
At a certain concentration of H2 and NH3, the initial rate of reaction is 0.120 M / s. What would the initial rate of the reaction be if the concentration of H2 were halved

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
At a certain concentration of H2 and NH3, the initial rate of reaction is 0.120 M / s. What would th...
Questions

Physics, 02.11.2020 18:10


History, 02.11.2020 18:10

Chemistry, 02.11.2020 18:10


English, 02.11.2020 18:10



Mathematics, 02.11.2020 18:10





Engineering, 02.11.2020 18:10

History, 02.11.2020 18:10

Mathematics, 02.11.2020 18:10

Mathematics, 02.11.2020 18:10



Biology, 02.11.2020 18:10

![rate=k[H_2]^2[NH_3]](/tpl/images/0537/2256/8fdd7.png)
![H_2 and [tex]I_2, the initial rate of reaction is 0.120 M/s. What would the initial rate of the reaction be if the concentration of [tex]H_2 were halved.Answer : The initial rate of the reaction will be, 0.03 M/sExplanation :Rate law expression for the reaction:[tex]rate=k[H_2]^2[NH_3]](/tpl/images/0537/2256/23b7f.png)
![0.120=k[H_2]^2[NH_3]](/tpl/images/0537/2256/be6ca.png) ....(1)
....(1)![R=k(\frac{[H_2]}{2})^2[NH_3]](/tpl/images/0537/2256/dca92.png) ....(2)
....(2)![\frac{R}{0.120}=\frac{k(\frac{[H_2]}{2})^2[NH_3]}{k[H_2]^2[NH_3]}](/tpl/images/0537/2256/6c355.png)