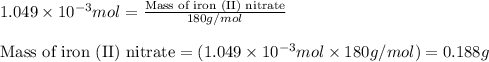Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
What minimum mass of iron (II) nitrate must be added to 10.0 of a 0.0699 M phosphate solution in ord...
Questions





Mathematics, 27.11.2021 05:10

Mathematics, 27.11.2021 05:10

Mathematics, 27.11.2021 05:10

Chemistry, 27.11.2021 05:10

French, 27.11.2021 05:10

Mathematics, 27.11.2021 05:10



Chemistry, 27.11.2021 05:10

History, 27.11.2021 05:20

History, 27.11.2021 05:20

Mathematics, 27.11.2021 05:20

English, 27.11.2021 05:20


Mathematics, 27.11.2021 05:20

Mathematics, 27.11.2021 05:20

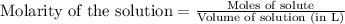

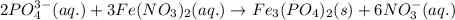
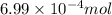 of phosphate solution will react with =
of phosphate solution will react with = 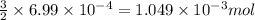 of iron (II) nitrate
of iron (II) nitrate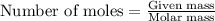
 moles
moles