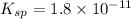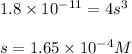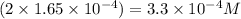Chemistry, 07.03.2020 02:24 avisconti571
A 350 mL saturated solution of magnesium hydroxide, Mg(OH)2 is prepared at 25 degrees Celsius. The solubility product constant, Ksp, for Mg(OH)2 is 1.8 x 10^-11 at 25 degrees Celsius.
1) Find the molar solubility of Mg(OH)2 at 25 degrees Celsius?
2) What is the concentration (mol/L) of hydroxide ion, OH-, in the saturated solution at 25 degrees Celsius?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

You know the right answer?
A 350 mL saturated solution of magnesium hydroxide, Mg(OH)2 is prepared at 25 degrees Celsius. The s...
Questions









Computers and Technology, 25.10.2019 23:43



Computers and Technology, 25.10.2019 23:43








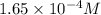
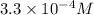
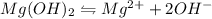
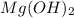 will be:
will be:![K_{sp}=[Mg^{2+}][OH^-]^2\\\\K_{sp}=s\times (2s)^2=4s^3](/tpl/images/0537/0995/7d1c0.png)