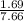Chemistry, 07.03.2020 02:47 garretthyatt123
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 200. mL flask with 2.6 atm of sulfur dioxide gas and 0.90 atm of oxygen gas at 35.°C. She then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 1.3 atm. Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions

Mathematics, 07.04.2021 21:20

Mathematics, 07.04.2021 21:20





Mathematics, 07.04.2021 21:20

Mathematics, 07.04.2021 21:20

Mathematics, 07.04.2021 21:20



English, 07.04.2021 21:20


Mathematics, 07.04.2021 21:20






History, 07.04.2021 21:20

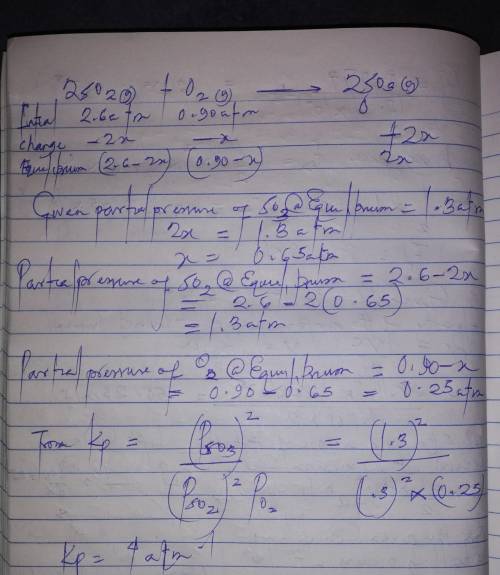
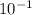 = 0.22 to 2 sig. fig.
= 0.22 to 2 sig. fig.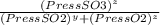
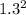 ÷ (
÷ (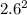 + 0.9)
+ 0.9)