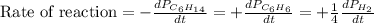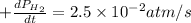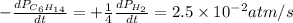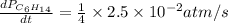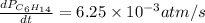Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

You know the right answer?
For the reaction C6H14(g) > C6H6(g) + 4H2(g), the rate of formation of hydrogen gas, H2 was found...
Questions


Geography, 13.02.2022 14:00


History, 13.02.2022 14:00




Arts, 13.02.2022 14:00

Mathematics, 13.02.2022 14:00




Social Studies, 13.02.2022 14:00

Mathematics, 13.02.2022 14:00



Mathematics, 13.02.2022 14:00



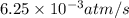
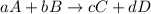
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0537/1900/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0537/1900/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0537/1900/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0537/1900/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0537/1900/d4b94.png)
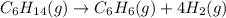
![\text{Rate of disappearance of }C_6H_{14}=-\frac{d[C_6H_{14}]}{dt}](/tpl/images/0537/1900/89957.png)
![\text{Rate of formation of }C_6H_6=+\frac{d[C_6H_6]}{dt}](/tpl/images/0537/1900/d8137.png)
![\text{Rate of formation of }H_2=+\frac{1}{4}\frac{d[H_2]}{dt}](/tpl/images/0537/1900/ecdf8.png)