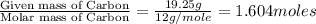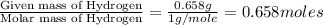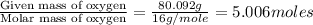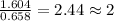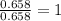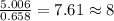Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

You know the right answer?
From combustion analysis, a 100.0 g sample of a compound that consists of C, H, and O was found to c...
Questions





Mathematics, 05.05.2020 18:22


Social Studies, 05.05.2020 18:22

History, 05.05.2020 18:22

Social Studies, 05.05.2020 18:22

Mathematics, 05.05.2020 18:22

Mathematics, 05.05.2020 18:22





Mathematics, 05.05.2020 18:22


Social Studies, 05.05.2020 18:22


Mathematics, 05.05.2020 18:22

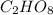
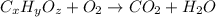
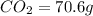
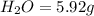
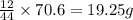 of carbon will be contained.
of carbon will be contained.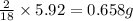 of hydrogen will be contained.
of hydrogen will be contained.