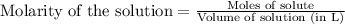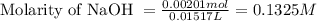Chemistry, 07.03.2020 02:37 loopysoop5035
A 0.411 g sample of potassium hydrogen phthalate, KHC8H4O4 (MM = 204.44 g/mol) is dissolved in 50 mL of deionized water in a 125-Erlenmeyer flask. The sample is titrated to the phenolphthalein endpoint with 15.17 mL of a sodium hydroxide solution. What is the molar concentration of the NaOH solution?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1
You know the right answer?
A 0.411 g sample of potassium hydrogen phthalate, KHC8H4O4 (MM = 204.44 g/mol) is dissolved in 50 mL...
Questions



Mathematics, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01



English, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01



English, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01




English, 16.10.2020 14:01


Physics, 16.10.2020 14:01

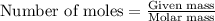
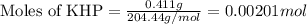

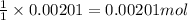 of NaOH.
of NaOH.