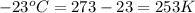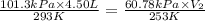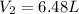Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Fugu, also known as puffer fish, is a sushi delicacy that can also be lethal. puffer fish contain a powerful toxin that can kill an adult a few hours after ingestion. sushi chefs who prepare fugu must be specially trained because any contamination of the toxin-free areas of the fish can be deadly. recently this toxin has been put to good use, as scientists have discovered that a purified form of it can treat severe pain in cancer patients. this recent scientific discovery would fall under which area of chemistry? applied biochemistry pure organic chemistry pure physical chemistry applied inorganic chemistry
Answers: 1

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
A helium balloon with an internal pressure of 1.0 atm and a volume of 4.50 L at 20.0⁰C is released....
Questions


Mathematics, 31.10.2020 05:50



Mathematics, 31.10.2020 05:50



Mathematics, 31.10.2020 05:50

Advanced Placement (AP), 31.10.2020 05:50

Computers and Technology, 31.10.2020 05:50




Mathematics, 31.10.2020 05:50


History, 31.10.2020 05:50




Mathematics, 31.10.2020 05:50

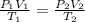
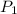 = initial pressure of gas = 1 atm = 101.3 kPa
= initial pressure of gas = 1 atm = 101.3 kPa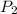 = final pressure of gas = 60.78 kPa
= final pressure of gas = 60.78 kPa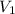 = initial volume of gas = 4.50 L
= initial volume of gas = 4.50 L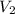 = final volume of gas = ?
= final volume of gas = ?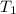 = initial temperature of gas =
= initial temperature of gas = 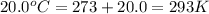
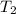 = final temperature of gas =
= final temperature of gas =