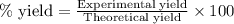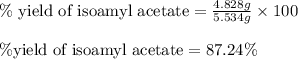Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
Suppose that in the synthesis of isoamyl acetate, a student began with 6.289 grams of acetic acid an...
Questions

Chemistry, 29.06.2019 19:30

History, 29.06.2019 19:30

History, 29.06.2019 19:30

Mathematics, 29.06.2019 19:30

History, 29.06.2019 19:30

Chemistry, 29.06.2019 19:30

English, 29.06.2019 19:30


English, 29.06.2019 19:30


Mathematics, 29.06.2019 19:30

Advanced Placement (AP), 29.06.2019 19:30

Computers and Technology, 29.06.2019 19:30


Computers and Technology, 29.06.2019 19:30


History, 29.06.2019 19:30

English, 29.06.2019 19:30

History, 29.06.2019 19:30

Mathematics, 29.06.2019 19:30

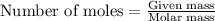 .....(1)
.....(1)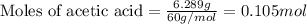
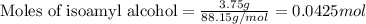
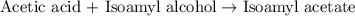
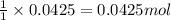 of acetic acid
of acetic acid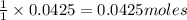 of isoamyl acetate
of isoamyl acetate