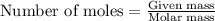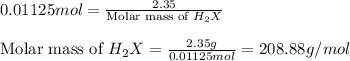Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 06:00
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control?
Answers: 1
You know the right answer?
A 2.35-g sample of an acid, H2X, requires 45.0 mL of a 0.500 M NaOH solution for complete reaction (...
Questions

Social Studies, 07.09.2021 19:00

Biology, 07.09.2021 19:00

Mathematics, 07.09.2021 19:00

Computers and Technology, 07.09.2021 19:00

Biology, 07.09.2021 19:00





Geography, 07.09.2021 19:00




Chemistry, 07.09.2021 19:00


Mathematics, 07.09.2021 19:00



Arts, 07.09.2021 19:00

Mathematics, 07.09.2021 19:00

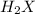 is 208.88 g/mol
is 208.88 g/mol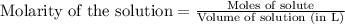
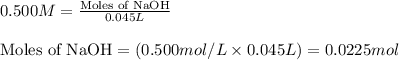
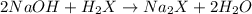
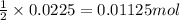 of
of