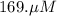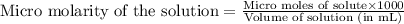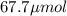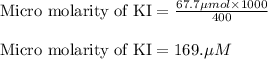Chemistry, 07.03.2020 03:40 ayoismeisalex
A chemist prepares a solution of potassium iodide (KI) by measuring out 67.7. mu mol of potassium iodide into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mmo1L of the chemist's potassium iodide solution. Round your answer to 3 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

You know the right answer?
A chemist prepares a solution of potassium iodide (KI) by measuring out 67.7. mu mol of potassium io...
Questions

English, 02.07.2019 21:30

History, 02.07.2019 21:30

Health, 02.07.2019 21:30


Mathematics, 02.07.2019 21:30


Mathematics, 02.07.2019 21:30





English, 02.07.2019 21:30



Chemistry, 02.07.2019 21:30

Biology, 02.07.2019 21:30




Mathematics, 02.07.2019 21:30