
Chemistry, 07.03.2020 04:14 mckleinrivero
The rate constant for a certain reaction is kkk = 3.50×10−3 s−1s−1 . If the initial reactant concentration was 0.450 MM, what will the concentration be after 19.0 minutes?
A zero-order reaction has a constant rate of 3.50×10−4 M/sM/s. If after 65.0 seconds the concentration has dropped to 3.50×10−2 MM, what was the initial concentration?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
The rate constant for a certain reaction is kkk = 3.50×10−3 s−1s−1 . If the initial reactant concent...
Questions



History, 10.03.2020 08:23

Social Studies, 10.03.2020 08:23

Computers and Technology, 10.03.2020 08:23

Computers and Technology, 10.03.2020 08:23




English, 10.03.2020 08:23

Mathematics, 10.03.2020 08:23


Mathematics, 10.03.2020 08:23







![Rate = k[A][B]](/tpl/images/0537/5969/ab111.png) . As the data are given in the question, the rate constant is
. As the data are given in the question, the rate constant is 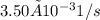 , the initial concentration
, the initial concentration![[A]_o = 0.450 M](/tpl/images/0537/5969/1d14c.png) and time is 19 minutes that is the 1140s.
and time is 19 minutes that is the 1140s.
![ln[A] = ln[A]_o - kt\\ln[A] = ln (0.450) - (3.50*10^-^3)1140\\ln[A] = -0.799 - 3.99\\ln[A] = -4.789\\[A] = 0.0083M](/tpl/images/0537/5969/91bfd.png)
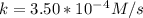 Time = 65 s
Final Concentration [A] =
Time = 65 s
Final Concentration [A] = 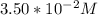
![[A] = [A]_o - kt[A]_o = [A] + kt\\[A]o = 3.50*10^{-2} + (3.50*10^{-4}) * 65\\[A]_o = 3.50*10^{-2} + 0.02275\\[A]_o = 0.05775 M](/tpl/images/0537/5969/3e5cb.png)


