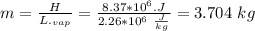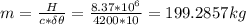Chemistry, 07.03.2020 03:56 damiangibson2
Assume that a daily diet of 2000 calories (i. e. 8.37 x 106 J) is converted completely to body heat.
a) How many g of water (as sweat) would need to evaporate to cool that person off?
b) If instead of evaporating water, the heat was used to raise the temperature of some water from 25.0 °C to 35.0 °C, how much water could be heated?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
Assume that a daily diet of 2000 calories (i. e. 8.37 x 106 J) is converted completely to body heat....
Questions

Mathematics, 15.02.2021 08:30

Arts, 15.02.2021 08:30

Mathematics, 15.02.2021 08:30

Mathematics, 15.02.2021 08:30



Mathematics, 15.02.2021 08:30


Biology, 15.02.2021 08:30


English, 15.02.2021 08:30

Chemistry, 15.02.2021 08:30

English, 15.02.2021 08:30


Physics, 15.02.2021 08:30

Mathematics, 15.02.2021 08:30


Mathematics, 15.02.2021 08:30