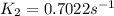Chemistry, 07.03.2020 04:09 beelcypher
The rate constant of a first-order reaction is 3.46 × 10−2 s−1 at 298 K. What is the rate constant at 350 K if the activation energy for the reaction is 50.2 kJ/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
The rate constant of a first-order reaction is 3.46 × 10−2 s−1 at 298 K. What is the rate constant a...
Questions

Social Studies, 25.02.2021 18:30

Mathematics, 25.02.2021 18:30

Computers and Technology, 25.02.2021 18:30


Mathematics, 25.02.2021 18:30

Mathematics, 25.02.2021 18:30




Mathematics, 25.02.2021 18:30

Mathematics, 25.02.2021 18:30



Biology, 25.02.2021 18:30


Mathematics, 25.02.2021 18:30



Mathematics, 25.02.2021 18:30

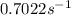 is the rate constant at 350 K if the activation energy for the reaction is 50.2 kJ/mol.
is the rate constant at 350 K if the activation energy for the reaction is 50.2 kJ/mol.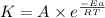
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0537/5727/6d953.png)
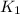 = rate constant at 298 K=
= rate constant at 298 K= 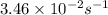
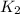 = rate constant at 350 K =?
= rate constant at 350 K =?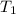 = initial temperature = 298 K
= initial temperature = 298 K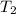 = final temperature = 350 K
= final temperature = 350 K![\log (\frac{K_2}{3.46\times 10^{-2} s^{-1}})=\frac{50200 J/mol}{2.303\times 8.314J/mole.K}[\frac{1}{350 K}-\frac{1}{298 K}]](/tpl/images/0537/5727/978f6.png)