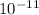Chemistry, 07.03.2020 04:02 hannahpalacios101
. Kc = 2.19*10-10 @ 100 oC for the rxn: COCl2(g) CO(g) + Cl2(g) the following mixtures may or may not be at equilibrium. Identify which mixtures are at equilibrium, if not, indicate which direction the reaction will go to obtain equilibrium (don’t guess, no credit given without supporting evidence). a) [CO] = 1.0*10-3, [Cl2] = 1.0*10-3, [COCl2]= 2.19*10-1 b) [CO] = 3.31*10-6, [Cl2] = 3.31*10-6, [COCl2]= 5.00*10-2 c) [CO] = 4.5*10-7, [Cl2] = 5.73*10-6, [COCl2]= 8.57*10-2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

You know the right answer?
. Kc = 2.19*10-10 @ 100 oC for the rxn: COCl2(g) CO(g) + Cl2(g) the following mixtures may or may...
Questions



History, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01

Chemistry, 05.10.2020 16:01



Mathematics, 05.10.2020 16:01


Biology, 05.10.2020 16:01

Geography, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01

Business, 05.10.2020 16:01

Mathematics, 05.10.2020 16:01



History, 05.10.2020 16:01



History, 05.10.2020 16:01

![\frac{[CO][Cl2]}{[COCl2]}](/tpl/images/0537/5342/fdabd.png)
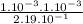 = 0.456.
= 0.456.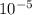
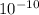 ), it can be deducted that it is not in equilibrium, since Kc1≠Kc and Kc1 is larger than Kc. When that occurs, it means the reaction is favoring the products, producing more of it. So, the equilibrium is going to the right.
), it can be deducted that it is not in equilibrium, since Kc1≠Kc and Kc1 is larger than Kc. When that occurs, it means the reaction is favoring the products, producing more of it. So, the equilibrium is going to the right.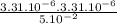 = 0.662.
= 0.662.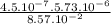 = 3.01.
= 3.01.