
Chemistry, 07.03.2020 05:13 danielburke24
Solution is prepared by adding 18 mL of 0.200 M Fe(NO3)3and 2 mL of 0.0020 M KSCN. Calculate the initial concentrations of Fe3 and SCN-in the solution

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

You know the right answer?
Solution is prepared by adding 18 mL of 0.200 M Fe(NO3)3and 2 mL of 0.0020 M KSCN. Calculate the ini...
Questions


Spanish, 23.05.2020 08:01

Mathematics, 23.05.2020 08:01




Mathematics, 23.05.2020 08:01

English, 23.05.2020 08:01


Mathematics, 23.05.2020 08:01


English, 23.05.2020 08:01


English, 23.05.2020 08:01

History, 23.05.2020 08:01





Mathematics, 23.05.2020 08:01

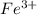 and
and 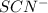 in the solution are, 0.200 M and 0.0020 M respectively.
in the solution are, 0.200 M and 0.0020 M respectively.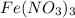 = 0.200 M
= 0.200 M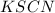 = 0.0020 M
= 0.0020 M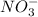 ion.
ion.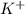 ion and 1 mole of
ion and 1 mole of 

