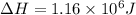Chemistry, 07.03.2020 05:14 spdesch2558
Calculate the amount of energy (in kilojoules) needed to heat 346 g of liquid water from –10 °C to 182°C. Assume that the specific heat of water is 4.184 J/g · °C for liquid and that the specific heat of steam is 1.99 J/g · °C.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Calculate the amount of energy (in kilojoules) needed to heat 346 g of liquid water from –10 °C to 1...
Questions

History, 01.09.2021 14:50

English, 01.09.2021 14:50

Mathematics, 01.09.2021 14:50



Social Studies, 01.09.2021 15:00


Mathematics, 01.09.2021 15:00

Social Studies, 01.09.2021 15:00

Geography, 01.09.2021 15:00

History, 01.09.2021 15:00

Social Studies, 01.09.2021 15:00

Mathematics, 01.09.2021 15:00

Mathematics, 01.09.2021 15:00


Mathematics, 01.09.2021 15:00

French, 01.09.2021 15:00

English, 01.09.2021 15:00


Social Studies, 01.09.2021 15:00

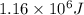
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+m\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+m\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0537/8966/4a4bb.png)
 = heat required for the reaction
= heat required for the reaction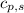 = specific heat of solid water or ice =
= specific heat of solid water or ice = 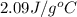
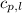 = specific heat of liquid water =
= specific heat of liquid water = 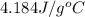
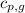 = specific heat of gaseous water =
= specific heat of gaseous water = 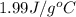
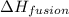 = enthalpy change for fusion =
= enthalpy change for fusion = 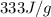
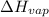 = enthalpy change for vaporization =
= enthalpy change for vaporization = 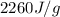
![\Delta H=[346g\times 2.09J/g^oC\times (0-(-80))^oC]+346g\times 333J/g+[346g\times 4.184J/g^oC\times (100-0)^oC]+346g\times 2260J/g+[346g\times 1.99J/g^oC\times (180-100)^oC]](/tpl/images/0537/8966/7d24b.png)