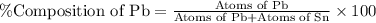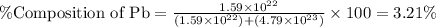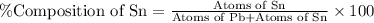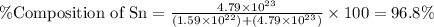Chemistry, 07.03.2020 04:57 fatherbamboo
What is the composition, in atom percent, of an alloy that consists of a) 5.5 wt% Pb and b) 94.5 wt% of Sn? Assume that the atomic weight for lead and tin are 207.2 and 118.71 g/mol, respectively.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 23:00
Need asap question 1 minerals are organic compounds. true false question 2 what vitamin can be found in foods like oranges, grapefruits, and broccoli? a. vitamin a b. vitamin k c.vitamin c d. vitamin d question 3 what are minerals? a. chemical elements that are needed for body processes. b. organic compounds that the body needs in small amounts to function properly. c. small molecules used to build proteins. d. an organic compound that is insoluble in water and includes fats. question 4 how many types of vitamins does the human body need? a. 15 b. 11 c. 13 d. 17 question 5 vitamins are a good source of energy. true false
Answers: 1

You know the right answer?
What is the composition, in atom percent, of an alloy that consists of a) 5.5 wt% Pb and b) 94.5 wt%...
Questions


Mathematics, 04.07.2019 01:00

Mathematics, 04.07.2019 01:00

History, 04.07.2019 01:00




Chemistry, 04.07.2019 01:00

History, 04.07.2019 01:00

Geography, 04.07.2019 01:00

Mathematics, 04.07.2019 01:00


Mathematics, 04.07.2019 01:00

Mathematics, 04.07.2019 01:00

Mathematics, 04.07.2019 01:00

Mathematics, 04.07.2019 01:00

Mathematics, 04.07.2019 01:00



Mathematics, 04.07.2019 01:00

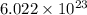 atoms
atoms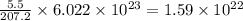 atoms
atoms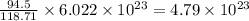 atoms
atoms