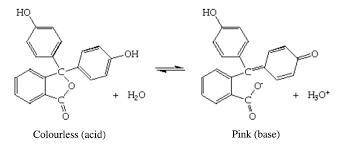
A student carried out an acid-base titration by adding NaOH solution from a buret to an Erlenmeyer flask containing HCl solution and using phenolphthalein as indicator. At the equivalence point, she observed a faint reddish-pink color. However, after a few minutes, the solution gradually turned colorless. What do you suppose happened?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
A student carried out an acid-base titration by adding NaOH solution from a buret to an Erlenmeyer f...
Questions




Computers and Technology, 22.10.2020 05:01

History, 22.10.2020 05:01


Mathematics, 22.10.2020 05:01


Biology, 22.10.2020 05:01

Social Studies, 22.10.2020 05:01

Mathematics, 22.10.2020 05:01


English, 22.10.2020 05:01

Engineering, 22.10.2020 05:01


Biology, 22.10.2020 05:01




Social Studies, 22.10.2020 05:01