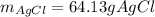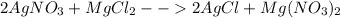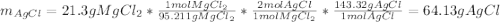Chemistry, 07.03.2020 04:49 izaiahfieods
When silver nitrate is added to an aqueous solution of magnesium chloride, a precipitation reaction occurs that produces silver chloride and magnesium nitrate. When enough AgNO3 is added so that 21.3 g of MgCl2 react, what mass of the AgCl precipitate should form

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
A48 g piece of ice at 0.0 ∘c is added to a sample of water at 7.4 ∘c. all of the ice melts and the temperature of the water decreases to 0.0 ∘c. how many grams of water were in the sample?
Answers: 1

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
You know the right answer?
When silver nitrate is added to an aqueous solution of magnesium chloride, a precipitation reaction...
Questions

Mathematics, 13.04.2020 20:45

History, 13.04.2020 20:45


English, 13.04.2020 20:45


History, 13.04.2020 20:46

Law, 13.04.2020 20:46



Chemistry, 13.04.2020 20:46

Mathematics, 13.04.2020 20:46


Biology, 13.04.2020 20:46

Biology, 13.04.2020 20:46

Chemistry, 13.04.2020 20:46

Mathematics, 13.04.2020 20:46

Mathematics, 13.04.2020 20:46


Mathematics, 13.04.2020 20:46

English, 13.04.2020 20:46