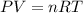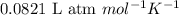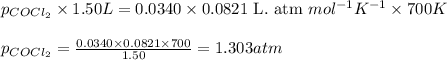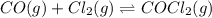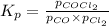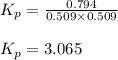Chemistry, 07.03.2020 04:27 fredorivera
Pure phosgene gas (COCl2), 0.0340 mol, was placed in a 1.50−L container. It was heated to 700.0 K, and at equilibrium, the pressure of CO was found to be 0.509 atm. Calculate the equilibrium constant KP for the reaction. CO(g) + Cl2(g) ⇌ COCl2(g)KP =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3


Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
Pure phosgene gas (COCl2), 0.0340 mol, was placed in a 1.50−L container. It was heated to 700.0 K, a...
Questions

Health, 21.11.2019 05:31

Chemistry, 21.11.2019 05:31

History, 21.11.2019 05:31


Arts, 21.11.2019 05:31





Physics, 21.11.2019 05:31

Mathematics, 21.11.2019 05:31

Mathematics, 21.11.2019 05:31


Mathematics, 21.11.2019 05:31

Computers and Technology, 21.11.2019 05:31

Health, 21.11.2019 05:31

Mathematics, 21.11.2019 05:31



Mathematics, 21.11.2019 05:31

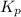 for the given equation is 3.065
for the given equation is 3.065