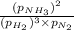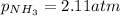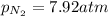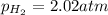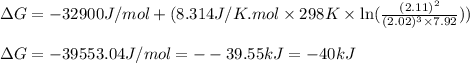Chemistry, 07.03.2020 04:48 Mattisback2285
A chemist fills a reaction vessel with 7.92 atm nitrogen (N2) gas, 2.02 atm hydrogen (H2) gas, and 2.11 atm ammonia (NH3) gas at a temperature of 25.0°C
Under these conditions, calculate the reaction free energy ΔG for the following chemical reaction
N2(8) +3H2 2NH3 (g)
Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
A chemist fills a reaction vessel with 7.92 atm nitrogen (N2) gas, 2.02 atm hydrogen (H2) gas, and 2...
Questions

Social Studies, 27.08.2019 13:20


History, 27.08.2019 13:20

History, 27.08.2019 13:20

History, 27.08.2019 13:20


Biology, 27.08.2019 13:20

Spanish, 27.08.2019 13:20

Advanced Placement (AP), 27.08.2019 13:20

Mathematics, 27.08.2019 13:20


Social Studies, 27.08.2019 13:20

English, 27.08.2019 13:20




Mathematics, 27.08.2019 13:20



Health, 27.08.2019 13:20

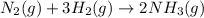
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(NH_3(g))})]-[(1\times \Delta G^o_f_{(N_2(g))})+(3\times \Delta G^o_f_{(H_2(g))})]](/tpl/images/0537/7676/6e73c.png)
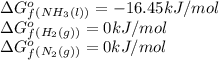
![\Delta G^o_{rxn}=[(2\times (-16.45))]-[(1\times (0))+(3\times (0))]\\\\\Delta G^o_{rxn}=-32.9kJ/mol](/tpl/images/0537/7676/8bdb6.png)
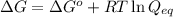
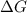 = free energy of the reaction
= free energy of the reaction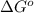 = standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)![25^oC=[273+25]K=298K](/tpl/images/0537/7676/0e82f.png)
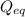 = Ratio of concentration of products and reactants at any time =
= Ratio of concentration of products and reactants at any time =