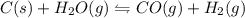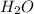Chemistry, 07.03.2020 04:48 katherine3084
Coal can be used to generate hydrogen gas (a potential fuel) by thefollowing endothermic reaction. C(s) + H2O (g) <==> CO(g) + H2(g)if this reaction mixture is at equilibrium, predict whether each ofthe following will result in the formation of additional hydrogengas, the formation of less hydrogen gas, or have no effect on thequantity og hydrogen gas. a. adding more C to the reaction mixtureb. adding more H2O to the reaction mixturec. raising the temperature of the reaction mixtured. increasing the volume of the reaction mixturee. adding a catalyst to the reaction mixturef. adding an inert gas to the reaction mixture

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
Coal can be used to generate hydrogen gas (a potential fuel) by thefollowing endothermic reaction. C...
Questions





Mathematics, 21.05.2020 02:57

Mathematics, 21.05.2020 02:57

Mathematics, 21.05.2020 02:57

English, 21.05.2020 02:57

Mathematics, 21.05.2020 02:57

Mathematics, 21.05.2020 02:57




Physics, 21.05.2020 02:57


Mathematics, 21.05.2020 02:57

Mathematics, 21.05.2020 02:57


Mathematics, 21.05.2020 02:57

Mathematics, 21.05.2020 02:57