Chemistry, 07.03.2020 04:28 pinapunapula
When methane (CH4) burns, it reacts with oxygen gas to produce carbon dioxide and water. The unbalanced equation for this reaction iCH4(g)+O2(g)→CO2(g)+H2O(g)This type of reaction is referred to as a complete combustion reaction. A)What mass of carbon dioxide is produced from the complete combustion of 1.10×10−3 g of methane?Express your answer with the appropriate units. B)What mass of water is produced from the complete combustion of 1.10×10−3 g of methane?Express your answer with the appropriate units. C)What mass of oxygen is needed for the complete combustion of 1.10×10−3 g of methane?Express your answer with the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1
You know the right answer?
When methane (CH4) burns, it reacts with oxygen gas to produce carbon dioxide and water. The unbalan...
Questions

Geography, 06.03.2022 21:30

Chemistry, 06.03.2022 21:30




World Languages, 06.03.2022 21:40

Mathematics, 06.03.2022 21:40



Mathematics, 06.03.2022 21:40

Business, 06.03.2022 21:40

Physics, 06.03.2022 21:40






Mathematics, 06.03.2022 21:40


Physics, 06.03.2022 21:40

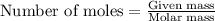 .....(1)
.....(1)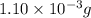
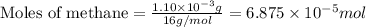
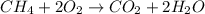
 of methane will produce =
of methane will produce =  of carbon dioxide
of carbon dioxide moles
moles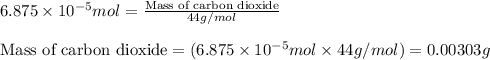
 of water
of water moles
moles