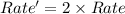Chemistry, 07.03.2020 04:28 blaze9889t
The reaction of methyl iodide with sodium azide, NaN3, proceeds by an SN2 mechanism. What is the effect of doubling the concentration of NaN3 on the rate of the reaction?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
The reaction of methyl iodide with sodium azide, NaN3, proceeds by an SN2 mechanism. What is the eff...
Questions



Arts, 14.02.2021 22:40


English, 14.02.2021 22:40

Mathematics, 14.02.2021 22:40



Mathematics, 14.02.2021 22:40



Biology, 14.02.2021 22:40

English, 14.02.2021 22:40

Mathematics, 14.02.2021 22:40



Biology, 14.02.2021 22:40

Mathematics, 14.02.2021 22:40

Computers and Technology, 14.02.2021 22:40

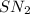 mechanism, both the reactants take part in the reaction and the rate law for the reaction will be:
mechanism, both the reactants take part in the reaction and the rate law for the reaction will be:![Rate=k[NaN_3]^1[CH_3I]^1](/tpl/images/0537/6635/f5055.png)
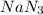 is doubled ,
is doubled , ![Rate'=k[2NaN_3]^1[CH_3I]^1](/tpl/images/0537/6635/8f46b.png)
![Rate'=2\times k[NaN_3]^1[CH_3I]^1](/tpl/images/0537/6635/f11c8.png)