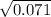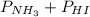Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
If Kp For This Reaction Is 0.071 At 100 °C (when The Partial Pressures Are Measured In Atmospheres),...
Questions

Mathematics, 02.01.2020 17:31

Mathematics, 02.01.2020 17:31

Physics, 02.01.2020 17:31

Mathematics, 02.01.2020 17:31


Biology, 02.01.2020 17:31

Mathematics, 02.01.2020 17:31


Mathematics, 02.01.2020 17:31

Social Studies, 02.01.2020 17:31

Mathematics, 02.01.2020 17:31

Mathematics, 02.01.2020 17:31



Physics, 02.01.2020 17:31

Biology, 02.01.2020 17:31

English, 02.01.2020 17:31


Mathematics, 02.01.2020 17:31

Mathematics, 02.01.2020 17:31

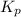 = 0.071 at 100°C
= 0.071 at 100°C![[P_{NH_3}][P_{HI}]](/tpl/images/0537/6634/19fd4.png)
![[x][x]](/tpl/images/0537/6634/ce5d5.png)