Chemistry, 07.03.2020 05:25 suttonfae336
Monochloroacetic acid (HC2H2ClO2) is a skin irritant that is used in "chemical peels" intended to remove the top layer of dead skin from the face and ultimately improve the complexion. The value of Ka for monochloroacetic acid is 1.35 ✕ 10−3. Calculate the pH of a 0.31 M solution of monochloroacetic acid.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
Monochloroacetic acid (HC2H2ClO2) is a skin irritant that is used in "chemical peels" intended to re...
Questions


English, 19.07.2019 07:30


Mathematics, 19.07.2019 07:30

Mathematics, 19.07.2019 07:30


Biology, 19.07.2019 07:30

Chemistry, 19.07.2019 07:30

Mathematics, 19.07.2019 07:30

Physics, 19.07.2019 07:30

Mathematics, 19.07.2019 07:30

Mathematics, 19.07.2019 07:30


Mathematics, 19.07.2019 07:30




Mathematics, 19.07.2019 07:30

Mathematics, 19.07.2019 07:30

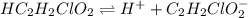
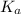 for above equation follows:
for above equation follows:![K_a=\frac{[H^+][C_2H_2ClO_2^-}}{[HC_2H_2ClO_2]}](/tpl/images/0537/9344/a55de.png)
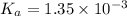
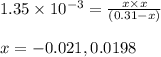
![pH=-\log[H^+]](/tpl/images/0537/9344/cf945.png)