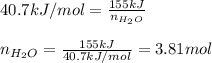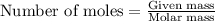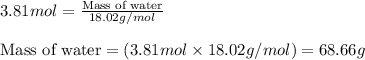Chemistry, 07.03.2020 05:07 moningersavannah
Calculate the mass of water (in g) that can be vaporized at its boiling point with 155 kJ of heat. • ΔHvap = 40.7 kJ/mol (at 100 °C) • 18.02 g H2O = 1 mol H2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
Calculate the mass of water (in g) that can be vaporized at its boiling point with 155 kJ of heat. •...
Questions

English, 20.07.2021 21:40



Mathematics, 20.07.2021 21:40



Mathematics, 20.07.2021 21:40


Mathematics, 20.07.2021 21:40

Mathematics, 20.07.2021 21:40

Mathematics, 20.07.2021 21:50




Mathematics, 20.07.2021 21:50

Mathematics, 20.07.2021 21:50

Biology, 20.07.2021 21:50



Mathematics, 20.07.2021 21:50

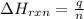
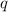 = amount of heat absorbed = 155 kJ
= amount of heat absorbed = 155 kJ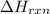 = enthalpy change of the reaction = 40.7 kJ/mol
= enthalpy change of the reaction = 40.7 kJ/mol