
Chemistry, 07.03.2020 05:04 wubzwaters
When 1328 J of heat energy is added to 40.4 g of ethanol, C 2 H 6 O , the temperature increases by 13.4 ∘ C. Calculate the molar heat capacity of C 2 H 6 O .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
When 1328 J of heat energy is added to 40.4 g of ethanol, C 2 H 6 O , the temperature increases by 1...
Questions



Mathematics, 01.11.2019 03:31

Chemistry, 01.11.2019 03:31

Chemistry, 01.11.2019 03:31

Biology, 01.11.2019 03:31














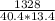 = 2.453 J/g·°C
= 2.453 J/g·°C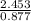 J/g·°C = 2.797 J/g·°C
J/g·°C = 2.797 J/g·°C

