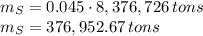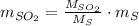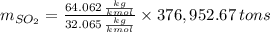Chemistry, 07.03.2020 05:01 qudoniselmore0
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 \rm tons of coal, a national record at that time.
Assuming that the coal was 86.0 \% carbon by mass and that combustion was complete, calculate the number of tons of carbon dioxide produced by the plant during the year.
Assuming that the coal was 4.50 \% sulfur by mass and that combustion was complete, calculate the number of tons of sulfur dioxide produced by the plant during the year.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2

Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1
You know the right answer?
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 \rm tons of coal, a nat...
Questions

Mathematics, 07.10.2020 19:01

Mathematics, 07.10.2020 19:01


Geography, 07.10.2020 19:01

Physics, 07.10.2020 19:01

English, 07.10.2020 19:01

Mathematics, 07.10.2020 19:01



History, 07.10.2020 19:01




Mathematics, 07.10.2020 19:01

Mathematics, 07.10.2020 19:01



Mathematics, 07.10.2020 19:01


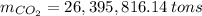 , b)
, b) 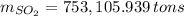
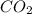 is produced by a mole of
is produced by a mole of 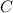 contained in coal. The yearly burnt carbon is:
contained in coal. The yearly burnt carbon is: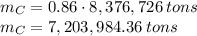
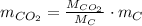
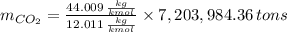
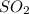 is produced by a mole of
is produced by a mole of 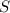 contained in coal.
contained in coal.