
Chemistry, 07.03.2020 05:21 juanitarodriguez
Calculate the entropy change when a. two moles of H2O(g) are cooled irreversible at constant p from 120°C to 100°C. b. one mole of H2O(g) is expanded at constant pressure of 2 bar from an original volume of 20 L to a final volume of 25 L. You can consider the gas to be ideal. c. one hundred grams of H2O(s) at -10°C and 1 bar are heated to H2O(l) at +10°C and 1 bar.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Which is true of chemicals? a. things containing chemicals always cost a lot of money. b. chemicals are never dangerous. c. chemicals are in many substances in a home. d. chemicals are rarely found on earth.
Answers: 1

Chemistry, 21.06.2019 15:30
Which of the following mining methods disrupts the sea floor?
Answers: 1


Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Calculate the entropy change when a. two moles of H2O(g) are cooled irreversible at constant p from...
Questions

History, 06.05.2020 15:02

English, 06.05.2020 15:02


History, 06.05.2020 15:02


Mathematics, 06.05.2020 15:02

Mathematics, 06.05.2020 15:02

Mathematics, 06.05.2020 15:02






Chemistry, 06.05.2020 15:02

Mathematics, 06.05.2020 15:02





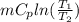 Where
Where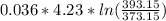 = 0.00795 kJ/(K)
= 0.00795 kJ/(K) 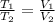 Therefore change in entropy S₂ - S₁ =
Therefore change in entropy S₂ - S₁ =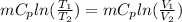
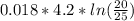 = -0.01686965 kJ/(K) = -16.9 J/K
= -0.01686965 kJ/(K) = -16.9 J/K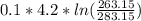 = -0.03078 kJ/K
= -0.03078 kJ/K

