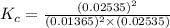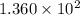Chemistry, 07.03.2020 05:42 jasminebrown72
Before any reaction occurs, the concentrations of A and B in the reaction below are each 0.03900 M . What is the equilibrium constant if the concentration of A at equilibrium is 0.01365 M ? 2A(aq)+B(aq)⇌2C(aq) Round your answer to one decimal place.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1
You know the right answer?
Before any reaction occurs, the concentrations of A and B in the reaction below are each 0.03900 M ....
Questions

History, 10.05.2021 21:20

Mathematics, 10.05.2021 21:20


History, 10.05.2021 21:20


Mathematics, 10.05.2021 21:20

Mathematics, 10.05.2021 21:20


Mathematics, 10.05.2021 21:20





Mathematics, 10.05.2021 21:20






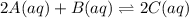
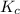 of the given reaction is as follows.
of the given reaction is as follows.![K_{c} = \frac{[C]^{2}}{[A]^{2}[B]}](/tpl/images/0538/0202/8ca5b.png)