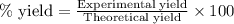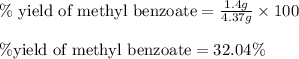Chemistry, 07.03.2020 05:25 livvyr0cks
Consider the Fischer ester synthesis of methyl benzoate from benzoic acid and methanol in the presence of sulfuric acid as a catalyst. A reaction was performed in which 3.6 g of benzoic acid was reacted with excess methanol to make 1.4 g of methyl benzoate. Calculate the theoretical yield and percent yield for this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2
You know the right answer?
Consider the Fischer ester synthesis of methyl benzoate from benzoic acid and methanol in the presen...
Questions


Chemistry, 24.02.2021 01:00

Social Studies, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

Health, 24.02.2021 01:00

English, 24.02.2021 01:00

Biology, 24.02.2021 01:00


Mathematics, 24.02.2021 01:00




Mathematics, 24.02.2021 01:00





Mathematics, 24.02.2021 01:00

Mathematics, 24.02.2021 01:00

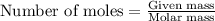 .....(1)
.....(1)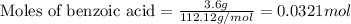
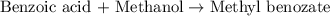
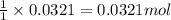 of methyl benzoate
of methyl benzoate