
Chemistry, 07.03.2020 05:36 damien1030
Calculate the pressures of NO, Cl2, and NOCl in an equilibrium mixture produced by the reaction of a starting mixture with 8.2 atm NO and 4.1 atm Cl2. (Hint: Kp is relatively large; assume the reaction goes to completion then comes back to equilibrium.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Calculate the pressures of NO, Cl2, and NOCl in an equilibrium mixture produced by the reaction of a...
Questions

Chemistry, 19.09.2019 12:20




Biology, 19.09.2019 12:20


Mathematics, 19.09.2019 12:20

Biology, 19.09.2019 12:20


Mathematics, 19.09.2019 12:20


Health, 19.09.2019 12:20

History, 19.09.2019 12:20





Mathematics, 19.09.2019 12:20


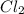 , and NOCl in an equilibrium mixture produced by the reaction of a starting mixture with 8.2 atm NO and 4.1 atm
, and NOCl in an equilibrium mixture produced by the reaction of a starting mixture with 8.2 atm NO and 4.1 atm 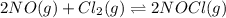
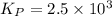
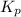 of the reaction is as follows.
of the reaction is as follows.![K_{P} = \frac{[NOCl]^{2}}{[NO]^{2}[Cl_{2}]}](/tpl/images/0537/9927/829f4.png)
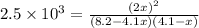
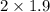 = 3.8
= 3.8

