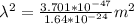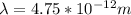A particle confined to rotate on a sphere is a very good approximation for the rotations ofdiatomic molecules. Using the equation for the energy of a particle rotating on a sphere, compute the equilibrium bond length of H137I, given that the rotational energy in the`= 5state is 8.952×10−21J. HINT: You will need to use the reduced mass.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
You know the right answer?
A particle confined to rotate on a sphere is a very good approximation for the rotations ofdiatomic...
Questions

Mathematics, 08.01.2021 19:50




History, 08.01.2021 19:50

Arts, 08.01.2021 19:50

Arts, 08.01.2021 19:50

Mathematics, 08.01.2021 19:50

Mathematics, 08.01.2021 19:50


History, 08.01.2021 19:50

Mathematics, 08.01.2021 19:50






Mathematics, 08.01.2021 19:50

History, 08.01.2021 19:50

English, 08.01.2021 19:50

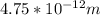
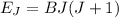
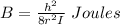
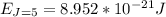
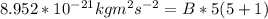
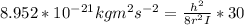
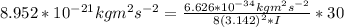
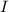 the subject
the subject 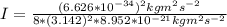
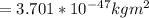
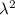
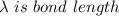
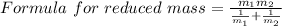
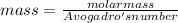
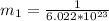
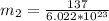

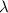 the subject of the formula
the subject of the formula