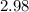Chemistry, 07.03.2020 06:17 missheather0309
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed explosively according to the following equation:
NH4NO3(s) N2O(g) + 2H2O(g)
At equilibrium, the total pressure in the container was found to be 2.72 bar at a temperature of 500.°C. Calculate Kp.
a.
1.64
b.
0.822
c.
2.98
d.
80.5
e.
0.745

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

You know the right answer?
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed...
Questions




Mathematics, 08.02.2021 21:00

Mathematics, 08.02.2021 21:00



Mathematics, 08.02.2021 21:00




Mathematics, 08.02.2021 21:00






Mathematics, 08.02.2021 21:00